A.D.A.M. - new standards of regenerative medicine
TECHNOLOGY
In August, 2020, FDA confirmed 510(k) eligibility for A.D.A.M. biopolymer and bioceramic bone implants. No human trials are required. FDA clearance is expected in 2024
Indications for use
Orthopedic surgery
CMF, neuro-, and plastic surgery
Oncology
STEP 1
Patients may upload and securely store their MRI and CAT scans on the digital platform. Should the patient be in need of the bone implant, certified clinicians would be able to use the platform to create a 3D model of the implant based directly on the patient's medical data.
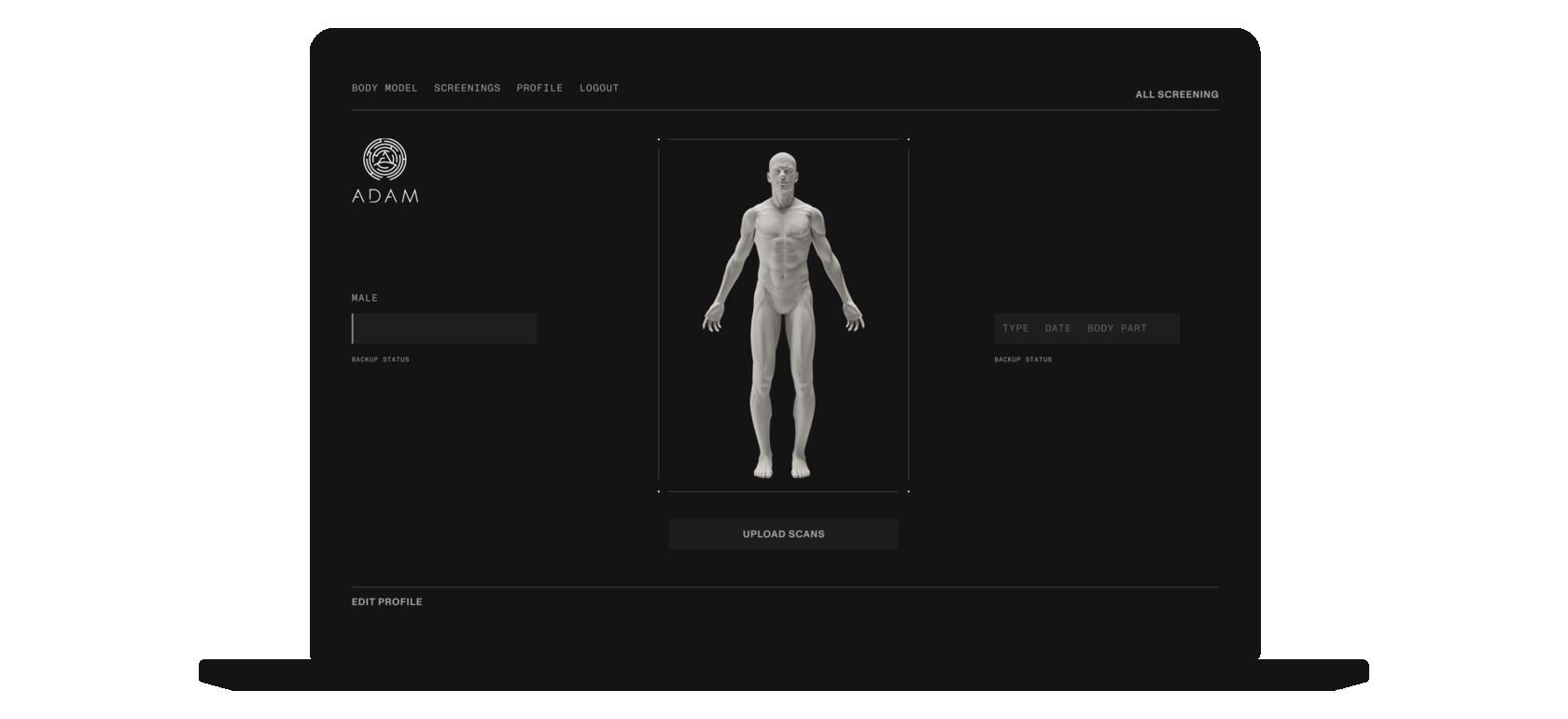
IMPLANT MODELING
STEP 2
IMPLANT PRODUCTION
The resulting 3D model of the patient-specific bone implant will be used for its subsequent production using A.D.A.M. proprietary 3D printers. Printing may take place at A.D.A.M. or directly on site, subject to the medical institution's preferences.
Overall production time will take up to 24 hours, which is substantially faster compared to the existing alternatives. At the same time, use of 3D-printing technology and A.D.A.M. material composites results in a lower cost of production than for titanium or PEEK-based implants.
Overall production time will take up to 24 hours, which is substantially faster compared to the existing alternatives. At the same time, use of 3D-printing technology and A.D.A.M. material composites results in a lower cost of production than for titanium or PEEK-based implants.
A personalized bone, just like yours.
With no toxic, allergic, and teratogenic effects.
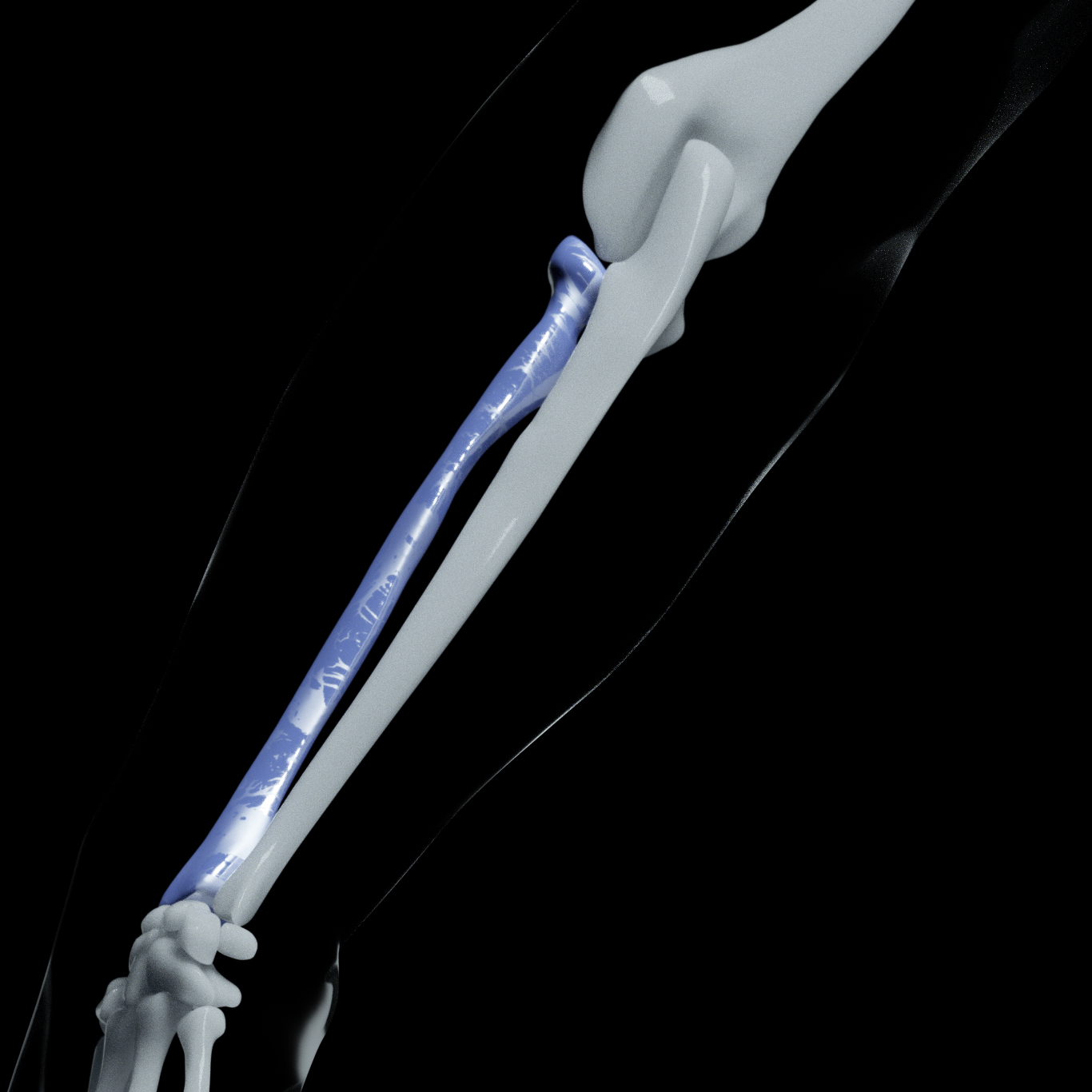
Modified biopolymer for tubular bone implants

Ceramic bio-glass for flat bone implants

- Provides high bioactivity
- Highly porous
- Stimulates osteogenesis
- Bioresorbable
Printing method: Binder Jetting Printing (BJP)
- Imitates natural bone structure
- Suitable for weight-bearing bones
- Stimulates osteogenesis
- Biodegradable
Printing method: Fused Filament Fabrication (FFF)
STEP 3
IMPLANTATION
A.D.A.M. will help medical professionals through a developed set of Quality Management System procedures, which will ensure safety, efficacy and quality of the implant.
Medical professionals will receive a sterilized 3D-printed bone implant ready for use in surgery.
Medical professionals will receive a sterilized 3D-printed bone implant ready for use in surgery.

STEP 4
RECOVERY
A.D.A.M. bone implants are biodegradable. They stimulate osteogenesis and are fully replaced by the bone tissue over time. Therefore, there is no need for reoperation to extract implant elements.
No hematoma, edema, scarring, degenerative changes, tumors, tissue necrosis and other abnormalities
Pre-clinical trials data:
Polymers
Ceramics
In the 4th week - cell migration and germination of the connective tissue (T) deep into the implant due to the porous structure of the ceramic material; in the 12th week - intensification of the process.
Formation of connective tissue capsule
Active phagocytic processes, partial destruction of test material and phagocytosis of cell metabolism products
local germination of connective tissue strands deep into the implanted material; increased neovascularization and formation of a large number of capillaries
- Pre-clinical trials completed in accordance with ISO standards
- Safety, biocompatibility and resorbability tested and confirmed
- FDA Q-submission response confirmed 510(k) rule eligibility
- No human trials required
- 2 PCT patents published (materials)
- Mount Sinai Innovation Partners' Elementa Labs startup


Bones are just the beginning. In future A.D.A.M. aims to deal with other tissues.
Expected market launch in 2024

A.D.A.M. mailing list
Stay up to date with A.D.A.M.'s progress and be the first to know when we go to market
Contact us
UConn Technology Incubation Program
9 W Broad St
Stamford, CT 06902
9 W Broad St
Stamford, CT 06902
93 Kanatna St
Odesa, Ukraine
65039
Odesa, Ukraine
65039
© 2021 ADAM • PRIVACY
3 Dorohozhytska St,
UnitCity B9, 2d floor
Kyiv, Ukraine
04078
UnitCity B9, 2d floor
Kyiv, Ukraine
04078