A.D.A.M. develops an on-demand personalized implant manufacturing infrastructure with a full scope of related services, provided remotely or on site
ABOUT


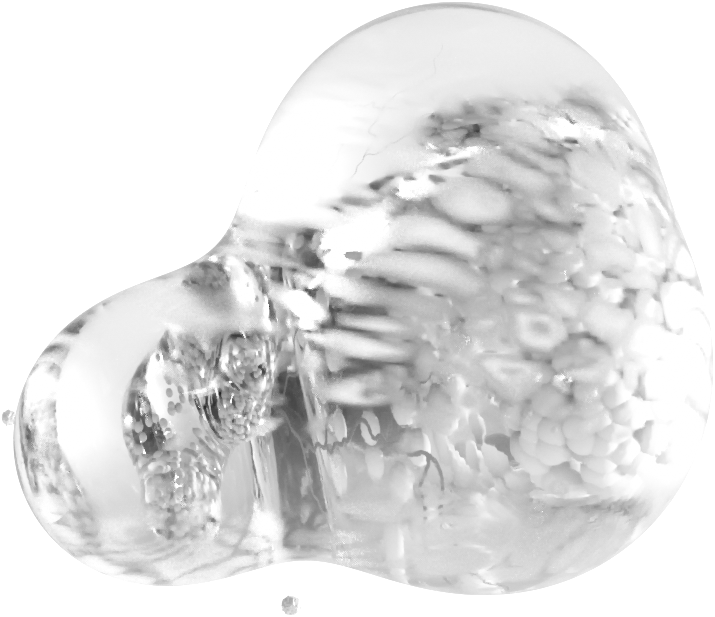
In August, 2020, FDA confirmed 510(k) eligibility for A.D.A.M. biopolymer and bioceramic bone implants. No human trials are required. FDA clearance is expected in 2022
In August, 2020, FDA confirmed 510(k) eligibility for A.D.A.M. biopolymer and bioceramic bone implants. No human trials are required. FDA clearance is expected in 2024.
A.D.A.M. was founded in 2018 by 3D printing company Kwambio and WeFund Ventures in Ukraine. The initial vision of the company was to create a carshop-like infrastructure where any part of the human body can be treated like a replaceable part. A.D.A.M. team decided to start the development of the project with a focus on bone implants and have developed proprietary 3D printing technology and material composites for the implants. The first prototypes passed pre-clinical trials in wistar rats. Successful results of the trials allowed A.D.A.M. to file the Q-Submission to the FDA. In their response, the FDA confirmed A.D.A.M. biopolymer and bioceramic bone implants' 510(k) eligibility. Concurrently, the company moved its HQs to the Technology Incubation Program at University of Connecticut.
The company had its first two PCT patents published in 2021. A.D.A.M. is also proud to have been accepted to Elementa Labs, an incubation program of Mount Sinai Innovation Partners.
A.D.A.M. plans to complete the animal studies, required by the FDA, in 2023. Next, the company aims to receive regulatory clearance for bone implants and begin the sales stage in 2024.
Bone implants are just the beginning - in the years to come A.D.A.M. aims to launch R&D of blood vessel, heart valve and bronchial implants.
The company had its first two PCT patents published in 2021. A.D.A.M. is also proud to have been accepted to Elementa Labs, an incubation program of Mount Sinai Innovation Partners.
A.D.A.M. plans to complete the animal studies, required by the FDA, in 2023. Next, the company aims to receive regulatory clearance for bone implants and begin the sales stage in 2024.
Bone implants are just the beginning - in the years to come A.D.A.M. aims to launch R&D of blood vessel, heart valve and bronchial implants.
MANAGEMENT TEAM
TEAM
Denys Gurak
CEO
CEO
Former top executive of industrial conglomerates; expert in global medical trials regulations
Engineer specializing in thermal processing and materials chemistry
Mykhailo Pluzhnyk
CTO
CTO
Ada Bovsunovsky
Operations Manager
Operations Manager
Formerly an analyst at venture fund and a hedge fund

Alexander Gamota
Board Member
Board Member
30+ years of experience in executive, ownership, consulting, and board roles leading to over $4B in value.
Yuliya Shapovalova
Head of Ukraine Office
Head of Ukraine Office
Legal counsel, startup manager in IT and investment projects

Markian Silecky, Esq.
General Counsel
General Counsel
Corporate Counsel; co-founder of multiple multinational entities

Special Operations Surgeon, orthopaedic specialist and retired Colonel
Carlton G Savory, M.D.
Chief Medical Officer
Chief Medical Officer
ADVISORY BOARD
Dr. Phillip Karber
Founding Chair, Chief Strategy Advisor
Founding Chair, Chief Strategy Advisor
Chair of the Griffith Leadership Center at the University of Michigan, formerly executive at Kaiser Permanente
Former DARPA Director, expert in innovative research and development
Tamir Harosh
Business Development Advisor
Business Development Advisor
Professional advisor, leads consulting teams for companies and individuals
Dan Edmonds-Waters
Co-Chair, Strategic Advisor
Co-Chair, Strategic Advisor
Dr. Anthony Tether
Chief Scientific Advisor
Chief Scientific Advisor
Strategic business advisor with expertise in law and go-to-market domains
Eugene Fedorchenko
Digital Strategy Advisor
Digital Strategy Advisor
Entrepreneur with executive and board positions in ICT and technology driven organizations
Den Burykin
Digital Strategy Advisor
Digital Strategy Advisor

President at Potomac Foundation, former Head of Strategy at Dept of Defense, strategic advisor to NATO
Howard Krain
Co-Chair, Strategic Advisor
Co-Chair, Strategic Advisor

Ambassador Pete Hoekstra
Chief Govt Affairs Advisor
Chief Govt Affairs Advisor
Former US Ambassador to the Netherlands, political advisor
Associate Professor at UConn, expert in complex fluids and 3D printing
Dr. Anson Ma
Chief Technology Advisor
Chief Technology Advisor

Global strategic communications and political advisor
Christopher Harvin
Chief International Advisor
Chief International Advisor
Fellow at American Board of Healthcare Executives, expert in sales strategy & marketing
R&D TEAM
Vadim Volkov, M.D.
Medical Consultant
Medical Consultant
Dr. Svitlana Kost
Head of Quality Assurance
Head of Quality Assurance
Oleg Rogankov
R&D Engineer
R&D Engineer
Materials physics, production technology, technological control
Medical applications, bio-engineering, ISO/ASTM protocols
Biotechnology and pharmaceutical research, quality control
Serhii Gladyr
Mechanical Design Engineer
Mechanical Design Engineer
Technical design, mechanical engineering, 3D printer specialist
Dr. Dmytro Larin
Electrical Engineer
Electrical Engineer
Professor, technology design and development, research and testing

A.D.A.M. mailing list
Stay up to date with A.D.A.M.'s progress and be the first to know when we go to market
PRESS RELEASES
Venture Beat
8 oct
Yahoo Finance
30 oct
Markets Insider
Dec 3
Venture Beat
Apr 3
PARTNERS
Long-established bioscience ecosystem
Technology Incubation Program at the University of Connecticut
Early-stage venture capital firm
Top-Tier law firm in the area of "FDA Law"
MSIP translates Mount Sinai discoveries and innovations into commercial healthcare products
Fortune 500 American manufacturer of industrial tools and household hardware
One of the biggest network of seed accelerators in US
Top 3D-printing company
Industry oriented center dedicated to pre-competitive research in additive manufacturing
Contact us
Serendipity Labs,
700 Canal St., 1st floor, Stamford, CT 06902, USA
700 Canal St., 1st floor, Stamford, CT 06902, USA
93 Kanatna St
Odesa, Ukraine
65039
Odesa, Ukraine
65039
© 2024 A.D.A.M. • PRIVACY
3 Dorohozhytska St,
UnitCity B9, 2d floor
Kyiv, Ukraine
04078
UnitCity B9, 2d floor
Kyiv, Ukraine
04078
BASICS
Why typography matters?
