By continuing you confirm our Privacy Policy and use of cookies
I accept
CREATE YOURSELF
A.D.A.M. is a developer of an on-demand personalized implant manufacturing infrastructure with a full scope of related services, provided remotely or on site.
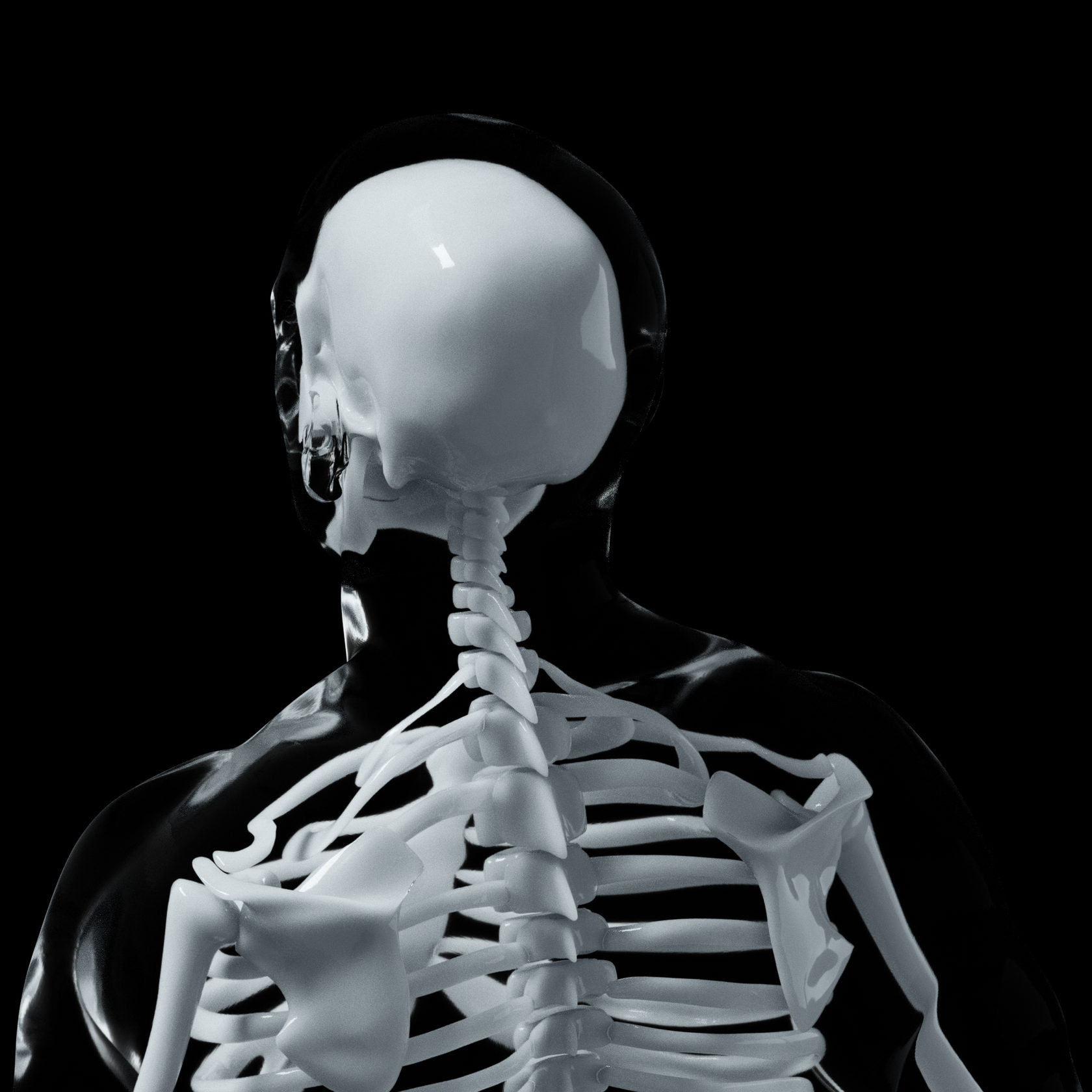
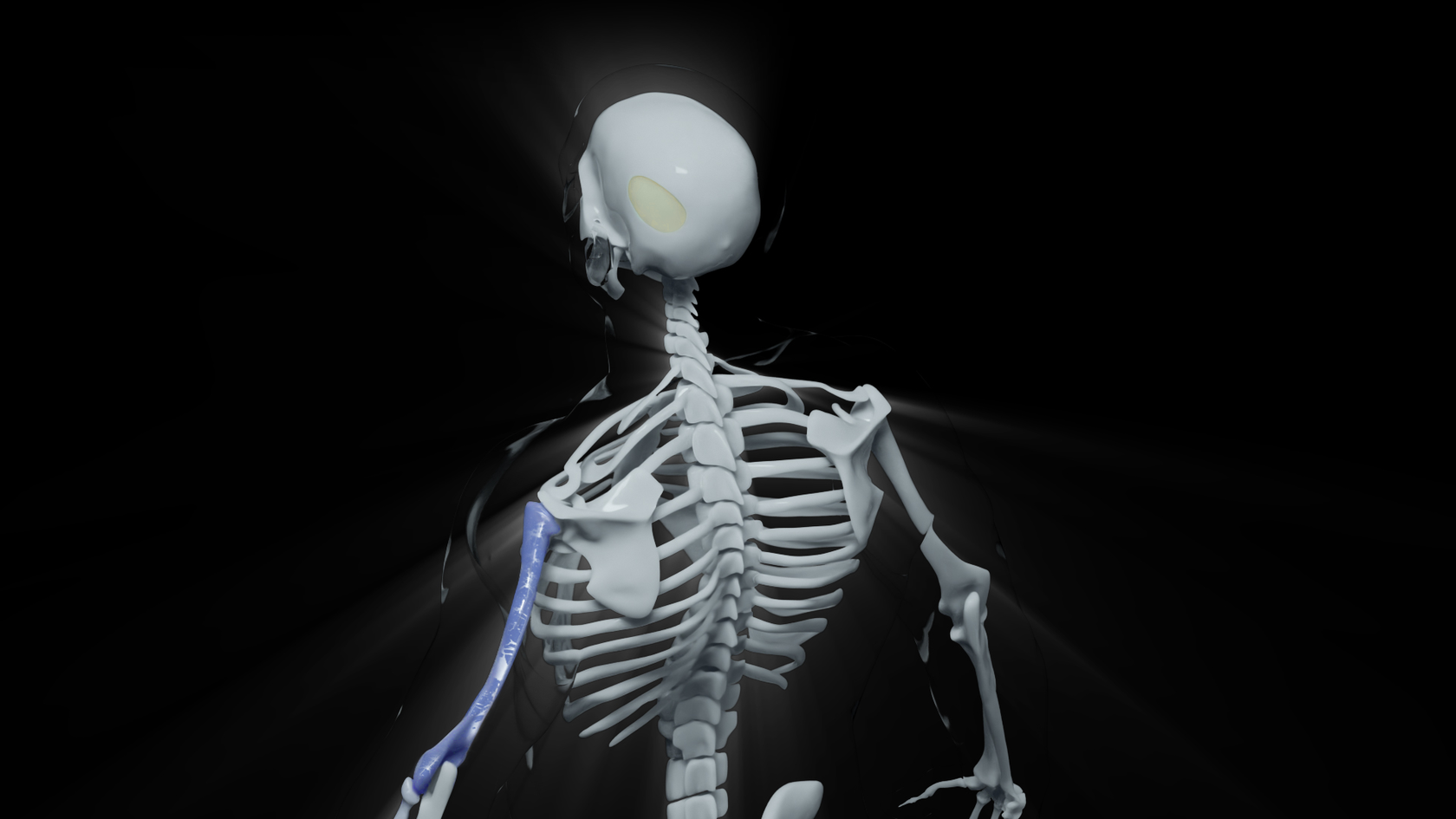
With A.D.A.M., medical professionals will be able to complete all steps of the implant-printing process from 3D-modeling to receiving a sterilized personalized device, ready for implantation.

A.D.A.M. mailing list
Stay up to date with A.D.A.M.'s progress and be the first to know when we go to market
Recently the global healthcare system problems have become strikingly evident. The COVID-19 outbreak has exacerbated existing issues in the industry, causing many professionals to rethink the healthcare models currently in place. Breakthrough improvements regarding treatment could surely result from big data and AI applications; however, the basic demand for materials (such as human tissue and organs), remains largely unmet due to supply chain vulnerabilities.
With the adoption of 3D printing in the healthcare industry, many of these problems could be drastically improved through cost reduction and time saving.
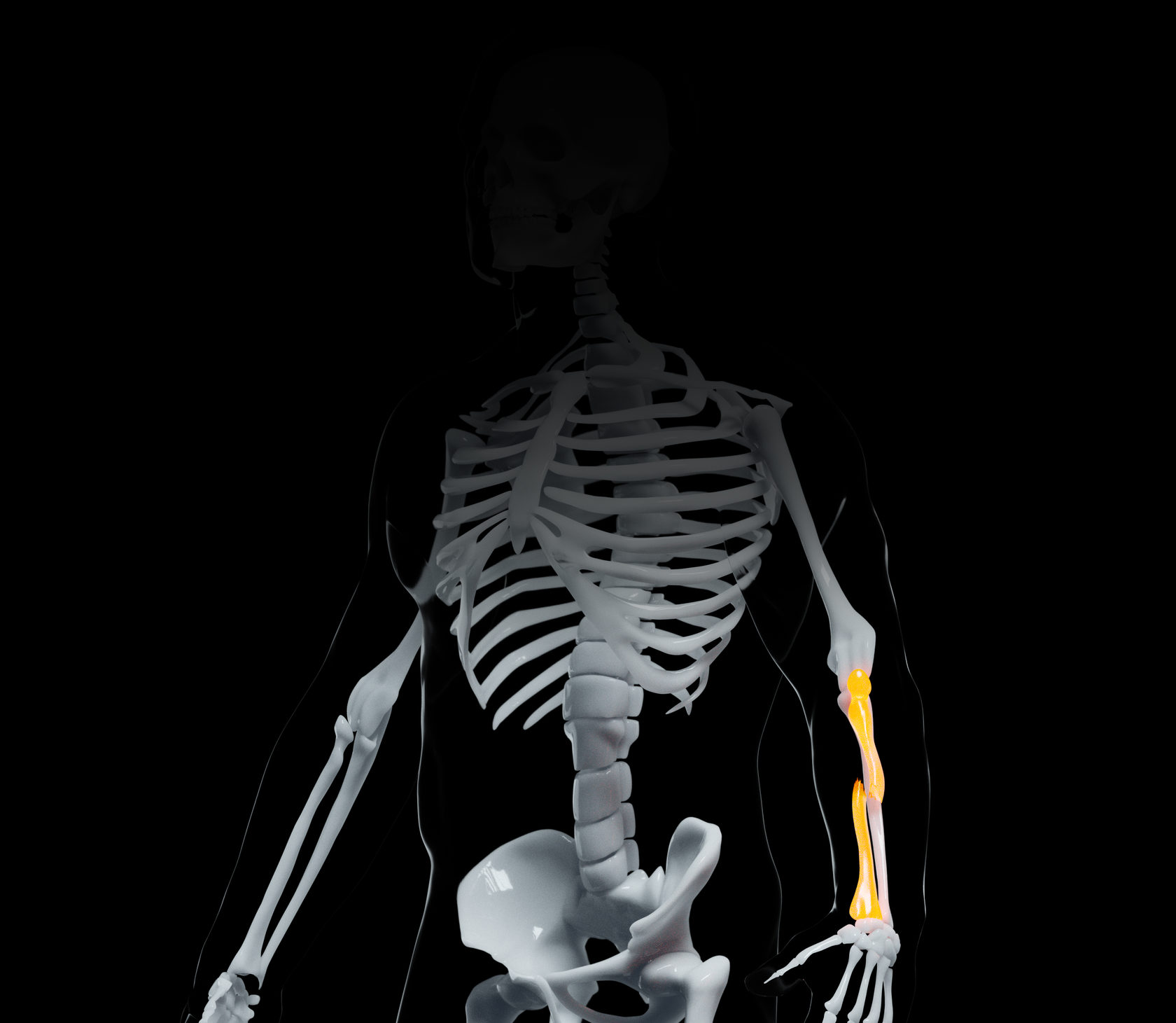
TECHNOLOGY-AS-A-SERVICE MODEL
By designing and building an on-demand tissue manufacturing solution, A.D.A.M. has been pioneering the technology as a service model to effectively address a complex problem. The business model will allow patients to have their tissue modelled – using the MRI/CAT scans stored on the digital platform – and, subsequently, 3D-printed and implanted in certified clinics.
STEP 1
Patients may upload and securely store their MRI and CAT scans on the digital platform. Should the patient be in need of the bone implant, certified clinicians would be able to use the platform to create a 3D model of the implant based directly on the patient's medical data.
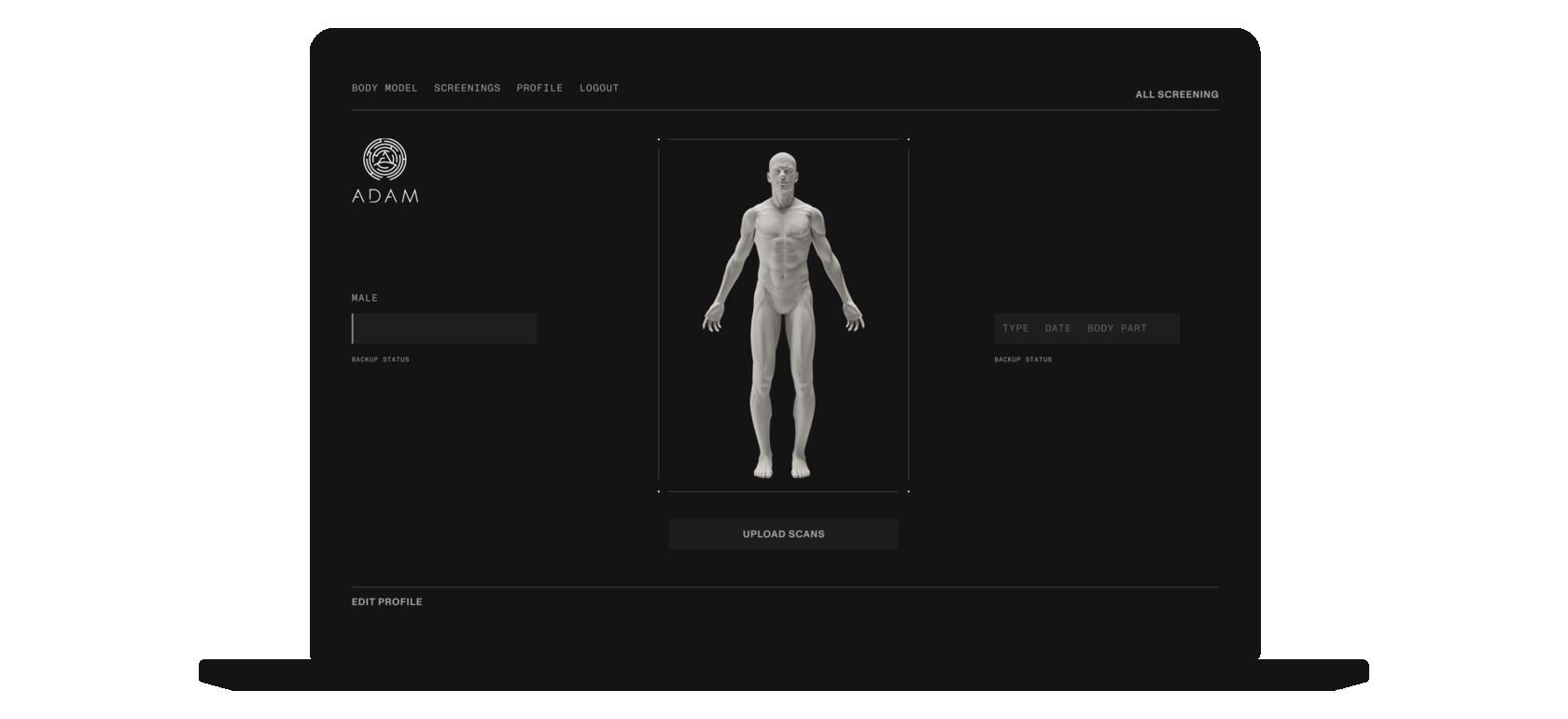
IMPLANT MODELING
STEP 2
IMPLANT PRODUCTION
The resulting 3D model of the patient-specific bone implant will be used for its subsequent production using A.D.A.M. proprietary 3D printers. Printing may take place at A.D.A.M. or directly on site, subject to the medical institution's preferences.
Overall production time will take up to 24 hours, which is substantially faster compared to the existing alternatives. At the same time, use of 3D-printing technology and A.D.A.M. material composites results in a lower cost of production than for titanium or PEEK-based implants.
Overall production time will take up to 24 hours, which is substantially faster compared to the existing alternatives. At the same time, use of 3D-printing technology and A.D.A.M. material composites results in a lower cost of production than for titanium or PEEK-based implants.
Flat Bone Implants

Tubular Bone Implants

Printing: Fused Filament Fabrication (FFF)
Material: ceramic bio-glass
Printing: Binder Jetting Printing (BJP)
Printing: Binder Jetting Printing (BJP)
STEP 3
IMPLANTATION
A.D.A.M. will help medical professionals through a developed set of Quality Management System procedures, which will ensure safety, efficacy and quality of the implant.
Medical professionals will receive a sterilized 3D-printed bone implant ready for use in surgery.
Medical professionals will receive a sterilized 3D-printed bone implant ready for use in surgery.

STEP 4
RECOVERY
A.D.A.M. bone implants are biodegradable. They stimulate osteogenesis and are fully replaced by the bone tissue over time. Therefore, there is no need for reoperation to extract metal fixation elements.
- Pre-clinical trials completed in accordance with ISO standards
- Safety, biocompatibility and resorbability tested and confirmed
- FDA Q-submission response confirmed 510(k) rule eligibility
- No human trials required
- 3 PCT patents published (materials & BJP printer)
- 1 provisional patent for FDM printer
- Mount Sinai Innovation Partners' Elementa Labs startup


MILESTONES
Bone implants are just the beginning - our goal is to offer a variety of tissues bioprinted on demand
Expected market launch in 2024
Contact us
Serendipity Labs,
700 Canal St., 1st floor, Stamford, CT 06902, USA
700 Canal St., 1st floor, Stamford, CT 06902, USA
93 Kanatna St
Odesa, Ukraine
65039
Odesa, Ukraine
65039
© 2024 A.D.A.M. • PRIVACY
3 Dorohozhytska St,
UnitCity B9, 2d floor
Kyiv, Ukraine
04078
UnitCity B9, 2d floor
Kyiv, Ukraine
04078

